Abstract
Background
Patients with newly diagnosed AML frequently present with abnormal organ function, poor performance status, concurrent active malignancies, and active infections. These factors often preclude these patients from enrollment on frontline clinical trials, since standard eligibility routinely exclude them. We previously studied a lower-intensity regimen of cladribine plus low-dose cytarabine (LDAC) alternating with decitabine (DAC) in older patients with newly diagnosed AML and demonstrated high rates of CR, excellent tolerability, and low early mortality. We conducted a phase II trial using this regimen for newly diagnosed patients with AML who were unfit or otherwise ineligible for existing clinical trials.
Methods
Patients >18 years with untreated AML and ineligible for other frontline clinical trials of AML therapy, were enrolled (NCT01515527). Eligibility criteria included either creatinine ≥2mg/dL or total bilirubin ≥2 mg/dL or ECOG Performance Status equal to 3 or 4 or ineligible for participation in a protocol of higher priority. Patients with active concurrent malignancies and ongoing infection related to their leukemia were allowed to be enrolled. Induction was cladribine 5 mg/m2 IV on D1-5, Cytarabine 20 mg SQ twice daily on days 1-10, followed by consolidation with cladribine 5 mg/m2 IV on D1-3, Cytarabine 20 mg SQ twice daily on days 1-10 alternating with decitabine 20 mg/m2 IV, daily on days 1-5. The primary objective was 60-d overall survival rate.
Results
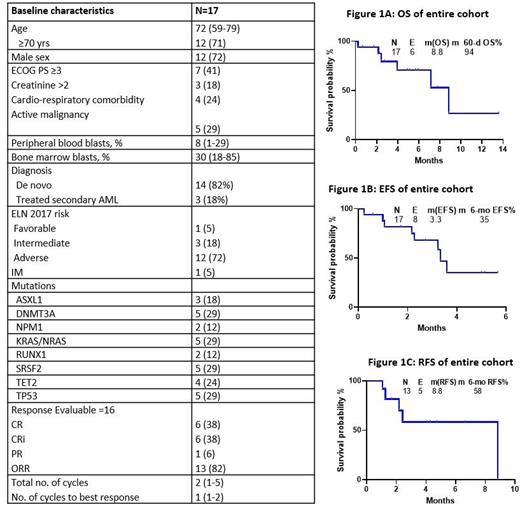
A total of 17 patients have been enrolled. The median age was 72 years (range, 59-79) with 71% of the cohort older than 70 years. Among the entire cohort, five (29%) had concurrent active malignancy, three pts (18%) had baseline creatinine >2mg/dL, and seven (41%) had ECOG PS ≥3. 72% of them were adverse risk per ELN 2017. Baseline characteristics are shown in Table 1.
The median follow up is 5.1 months (range, 0.7- 13.6 m). Among the 17 pts, 1 pt is too early for assessment and among the 16 evaluable pts , 1 pt (6%) died within six days of starting therapy, 13 (82%) achieved a response, including 6 (38%) complete remission (CR), 6 (38%) CR with incomplete count recovery (CRi) , and 1 (6%) partial response (Table 1). Of the 2 pts that did not respond, both were ELN adverse risk. Among the responders, 5 pts (33%) achieved MRD negativity by flow cytometry. Median cycles to response was 1 (range: 1 - 2) and patients had received a median of 2 cycles (range: 1 - 5).
Among the seventeen pts, 6 pts (35%) died, 2 (12%) were switched to different treatment regimens for lack of response, 7 (41%) continue to receive treatment, and 2 (12%) responders have been transitioned to maintenance therapy. Among the 6 deaths, 2 died on protocol with 1 death due to pseudomonal sepsis and the other due to pneumonia at 4 and 9 weeks of starting treatment, respectively. 2 pts (12%) died in CRi and 2 pts (12%) relapsed after an initial response and succumbed to AML. Median duration of response for the entire cohort is not reached. Median OS was 8.8 months, with 2- and 6-month OS rates of 94% and 71 %, respectively (Figure 1A). Median event free survival (EFS) is 3.3 months, with 2- and 6-month EFS rates of 82% and 35%, respectively (Figure 1B). Median relapse -free survival (RFS) is 8.8 months, with 2- and 6-month RFS rates of 81% and 58%, respectively (Figure 1C). Overall, the regimen was well tolerated, with an acceptable toxicity profile. Tumor lysis syndrome was not seen. There was no grade ≥3 adverse events related to therapy.
Conclusion
In an unfit patient population with a high comorbidity burden that were ineligible for other clinical trials, lower-intensity therapy with Cladribine plus LDAC was safe and effective in newly diagnosed pts with AML. The regimen produced high rates of response, encouraging event-free and overall survival, and low early mortality in a cohort predicted to have a high risk of early death. Treating this patient population on a clinical trial is feasible and can allow patients to achieve remission and move on to effective post-remission therapy.
Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Pemmaraju: Incyte: Consultancy; Affymetrix: Consultancy, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Springer Science + Business Media: Other; DAVA Oncology: Consultancy; CareDx, Inc.: Consultancy; Daiichi Sankyo, Inc.: Other, Research Funding; Roche Diagnostics: Consultancy; Celgene Corporation: Consultancy; Plexxicon: Other, Research Funding; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; MustangBio: Consultancy, Other; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; LFB Biotechnologies: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Sager Strong Foundation: Other; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Daver: Bristol Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Hanmi: Research Funding; Abbvie: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Amgen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Jain: Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Genentech: Honoraria, Research Funding; Janssen: Honoraria; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Pfizer: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Servier: Honoraria, Research Funding; TG Therapeutics: Honoraria; ADC Therapeutics: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Beigene: Honoraria; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. Burger: Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Alvarado: MEI Pharma: Research Funding; BerGenBio: Research Funding; Daiichi-Sankyo: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Consultancy, Research Funding; FibroGen: Research Funding; CytomX Therapeutics: Consultancy. DiNardo: Bristol Myers Squibb: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Novartis: Honoraria; Forma: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Borthakur: Protagonist: Consultancy; GSK: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; ArgenX: Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; Astex: Research Funding; Ryvu: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Ravandi: AstraZeneca: Honoraria; Amgen: Honoraria, Research Funding; Prelude: Research Funding; AbbVie: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Novartis: Honoraria; Xencor: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kantarjian: Ascentage: Research Funding; BMS: Research Funding; Astra Zeneca: Honoraria; Immunogen: Research Funding; Jazz: Research Funding; Novartis: Honoraria, Research Funding; Astellas Health: Honoraria; Aptitude Health: Honoraria; Precision Biosciences: Honoraria; KAHR Medical Ltd: Honoraria; NOVA Research: Honoraria; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Ipsen Pharmaceuticals: Honoraria; Pfizer: Honoraria, Research Funding; Taiho Pharmaceutical Canada: Honoraria. Kadia: Ascentage: Other; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Amgen: Other: Grant/research support; Pfizer: Consultancy, Other; Novartis: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Liberum: Consultancy; Dalichi Sankyo: Consultancy; Pulmotech: Other; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support; Sanofi-Aventis: Consultancy; AstraZeneca: Other; Astellas: Other; Genfleet: Other; Cellonkos: Other.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal